- 2022-08-12 发布 |
- 37.5 KB |
- 11页


申明敬告: 本站不保证该用户上传的文档完整性,不预览、不比对内容而直接下载产生的反悔问题本站不予受理。
文档介绍
生物学导论翻译
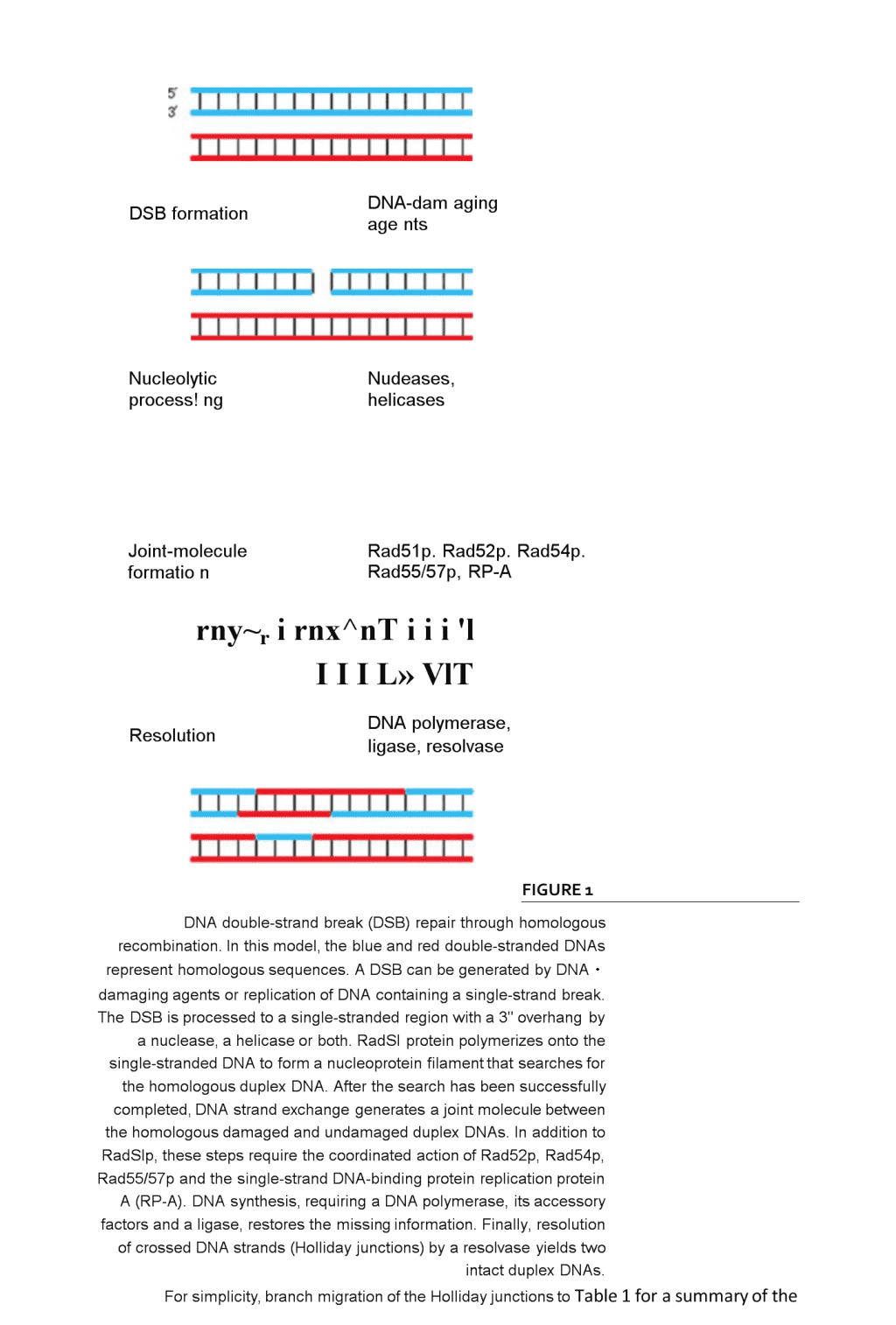
MolecularmechanismsofDNAdouble・strandbreakrepairRolandKanaar,JanH.J.HoeijmakersandDikC.vanGenDNAdouble・strandbreaks(DSBs)aremajorthreatstothegenomicintegrityofcells.Ifnottakencareofproperly,theycancausechromosomefragmentation,lossandtranslocation,possiblyresultingincarcinogenesis.UponDSBformation,cell-cyclecheckpointsaretriggeredandmultipleDSBrepairpathwayscanbeactivated.RecentresearchontheNijmegenbreakagesyndrome,whichpredisposespatientstocancer,suggestsadirectlinkbetweenactivationofcell-cyclecheckpointsandDSBrepair.Furthermore,thebiochemicalactivitiesofproteinsinvolvedinthetwomajorDSBrepairpathways,homologousrecombinationandDNAend-joining,arenowbeginningtoemerge.ThisreviewdiscussesthesenewfindingsandtheirimplicationsforthemechanismsofDSBrepair.TheintegrityofDNAinsidecellsisconstantlybeingchallengedbyendogenousandexogenousDNA-damagingagents.BecausealargevarietyoflesionscanoccurinDNA,itisnotsurprisingthatmultiplepathwayshaveevolvedthateachrepairasubsetoftheselesions1•ThesignificaneeofDNArepairisillustratedbythephenotypesofxerodermapigmentosum,Cockayne'ssyndrome,trichothiodystrophyandhereditarynonpolyposiscolorectalcancerpa2,3.ThesedisordersarecausedbymutationsinDNArepairgenesthatpredisposethepatientstocancer,neurologicalabnormalitiesorboth.Inaddi-tiontoefficientDNArepair,correctactivationofcell-cyclecheckpointsuponinductionofDNAdamageisofcrucialimportaneeforthemaintenanceofgenomicintegrity.CheckpointsallowactivelydividingcellstopauseandrepairDNAdamagebeforesegregationofthereplicatedgenomeintodaughtercells.TheirimportaneeisunderscoredbyinheriteddisordersassociatedwithdefectsinactivatingcellcyclecheckpointssuchasataxiatelangiectasiaandNijmegenbreakagesyndrome4,5.ThesedisorderscausehypersensitivitytoDNA-damagingagentsandspontaneouschromosomalinstability.Inthisreview,wefocusonmechanismsofDNAdouble-strandbreak(DSB)repairDSBsaregeneratedbyendogenouslyproducedradicalsandexogenousagentssuchasionizingradiation(IR),whichisoftenusedinanti-cancertherapy・RepairofDSBsisofcardinalimportaneetopreventchromosomalfragmentatio①translocationsand\ndeletions.Inthesoma,thegenomicinstabilityresultingfrompersistentorincorrectlyrepairedDSBscanleadtocarcinogenesisthroughactivationofoncogenes,inactivationoftumour-suppressorgenesorlossofheterozygosity,whileinthegermlinetheycanleadtoinborndefects.ThedeleteriouseffectsofDSBshavetriggeredtheevolutionofmultiplepathwaysfortheirrepair6,7.Homologousrecombinationrequiresextensivere・gionsofDNAhomologyandrepairsDSBsaccuratelyusinginformationontheundamagedsisterchromatidorhomologouschromosome・DNAend-joining,ontheotherhand,usesno,orextremelylimited,sequeneehomologytorejoinjuxtaposedendsinamannerthatneednotbeerrorfree(Figs1and2;see\nDSBformationDNA-damagingagentsNucleolyticprocess!ngNudeases,helicasesJoint-moleculeformationRad51p.Rad52p.Rad54p.Rad55/57p,RP-Arny~rirnx^nTiii'lIIIL»VlTResolutionDNApolymerase,ligase,resolvaseFIGURE1DNAdouble-strandbreak(DSB)repairthroughhomologousrecombination.Inthismodel,theblueandreddouble-strandedDNAsrepresenthomologoussequences.ADSBcanbegeneratedbyDNA・damagingagentsorreplicationofDNAcontainingasingle-strandbreak.TheDSBisprocessedtoasingle-strandedregionwitha3"overhangbyanuclease,ahelicaseorboth.RadSIproteinpolymerizesontothesingle-strandedDNAtoformanucleoproteinfilamentthatsearchesforthehomologousduplexDNA.Afterthesearchhasbeensuccessfullycompleted,DNAstrandexchangegeneratesajointmoleculebetweenthehomologousdamagedandundamagedduplexDNAs.InadditiontoRadSlp,thesestepsrequirethecoordinatedactionofRad52p,Rad54p,Rad55/57pandthesingle-strandDNA-bindingproteinreplicationproteinA(RP-A).DNAsynthesis,requiringaDNApolymerase,itsaccessoryfactorsandaligase,restoresthemissinginformation.Finally,resolutionofcrossedDNAstrands(Hollidayjunctions)byaresolvaseyieldstwointactduplexDNAs.Forsimplicity,branchmigrationoftheHollidayjunctionstoTable1forasummaryofthe\npropertiesoftheproteinsinvolvedandthecorrespondingmutantphenotypes).GeneticanalysesoftheeffectsofIRonSaccharomycescerevisiaeandrodentcellshavebeencrucialinidentifyinggenesinvolvedinDSByeastmutantsweredefectiveinhomologousrecombination,whereasthemutantrodentcelllinesweredefectiveinDNAend-joining.However,recentstudieshaveshownthatbothpathwayscontributetoDSBrepairinyeastandmammalsbutthattheirrelativecontributioncanvary(seebelow).ThegeneticanalysesarenowbeingcomplementedbythebiochemicalcharacterizationoftheDSBrepairpathways・DSBrepairbyhomologousrecombinationGeneticanalysesinS.cerevisiaehaveledtotheidentificationofgenesrequiredforDSBrepairbyhomologousrecombination9,12.Screensforradiation・sensitiveormeioticrecombination-deficientyeastcellshaverevealedtheinvolvementoftheRAD52epistasisgroupgenes(RAD50,RAD51,RAD52,RAD54,RAD55,RAD57,RAD59,MREllandXRS2).TheimportanceoftheRAD52recombi・nationalDNArepairpathwayisunderscoredbyitsevolutionaryconservation.MouseandhumangeneswithsequencesimilaritytoRAD50,RAD51,RAD52,RAD54andMREllhavebeenisolated7,10・Theavail-abilityoftheRAD52groupgeneshasprovidedtheessentialtoolsforbiochemicalanalysesofthemolecularmechanismofhomologousrecombination.TheaminoacidsequenceofS.cerevisiaeRad51pguidedtheinitialbiochemicalexperimentsbecauseofitssimilaritytotheEscherichiacolirecombinationproteinRecA6•Theseexperimentsshowedthatkeyreactionsinhomologousrecombination一thesearchforhomologousDNAandDNAstrandexchangeareperformedbyremarkablysimilarmechanismsinbacteria,yeastandhumancells.RecA,Rad51pandhumanRAD51polymerizeonDNAtoformanucleoproteinfilamentthatsearchesforhomologousDNA13•ThissearchendswiththeformationofjointmoleculesbetweenthehomologousDNAs,followedbyexchangeofDNAstrands(seeFig.1).TheS.cerevisiaeproteinsRad55pandRad57pdisplaysequeneesimilaritytoRad51p・However;itisunlikelythatthesearefunctionalhomologuesofRad51psincethereisnoevidenceforRad55porRad57pnucleoproteinfilamentformation.Instead,thesetwoproteinsformaheterodimerthatcanstimulateRad51p-mediatedstrandexchangeatsub-stoichiometricamountsrelativetoRad51p14.ThereareseveralotherproteinswithsimilaritytoRadSlpinmammalsaswell.SincedisruptionofmouseRAD51resultsinembryoniclethality6,theothermammalianproteinswithsequencesimilaritytoRAD51(RAD51B,RAD51C,RAD51D,XRCC2andlossofmouseRAD51functionXRCC3)areapparentlyunabletosubstituteforthe15-21•Atpresent,itisunclearwhethertheseproteinscanformnucleopro-einfilaments,likeRAD51,orwhethertheyfunctionanalogouslytoRad55/57p.ThereareseveralreasonswhymultipleRAD51-likeproteinsthatcanformnucleoproteinfilamentsmightberequiredinmammals.•Theycouldassembleseparatenucleoproteinfila-mentsthathavespec讦iccharacteristicstofacilitaterepairinpartsofthegenomethatdifferinchromatinstructureorcontainspecialsequencessuchastelomeresorcentromeres.Inaddition,nucleoproteinfilamentswithslightlydifferentpropertiesmightberequiredtorepairdifferenttypesofDNAdamage,suchasDSBsorinterstrandDNAcrosslinks.•Theycouldformmixedfilamentsinwhichthedifferentproteinshavespecificfunctio\nns.Forexample,aRAD51-likeprotomercouldbeincor・poratedintheRAD51nucleoproteinfilamentatsubstoichiometriclevelstoserveasadockingsiteforotherproteinsthatneedtointeractwiththefilament,butnotwitheveryRAD51protomer.•Theycouldberequiredwhenthehomologouschromosomeinsteadofthesisterchromatidisusedasatemplate.•Theproteinscouldhavetissue-specificfunctions.ThestepsthatleadtoS.cerevisiaeRad51p-mediatedjoint-moleculeformationcanbestimulatedbyovercomeinhibitoryeffectsofcompetingreactionsinthecellInadditiontoRad55/57p,Rad52pplaysacrucialroleinstimulatingRadSlp.BiochemicalanalysesofRad52phaveshownthatitpromotesannealingofcomplementarysingle-strandedDNAs22andfunctionsasamediatorbetweenRadSlpandthesingle-strandDNA-bindingproteinRP-Atostimu-lateRad51p-mediatedDNAstrandexchange14,23,24Similarly,humanRAD52proteinassistshumanRAD51informingjointmolecules25•However,thephenotypeofRAD52mutationsinS.cerevisiae,fissionyeastSchizosaccharomycespombeandmousecellsdiffersdramatically.WhiletheefficiencyofrecombinationisreducedbymorethanthreeordersofmagnitudeintheS.cerevisiaerad52mutant,itisonlytwo・foldlowerinthecorrespondingS・pombemutantandinmouseRAD52-knockoutembryonicstemcells7,26.TheproteinencodedbytheS.cerevisiaeRAD59geneshowssequeneesimilaritytoRad52p27.AdditionalhomologuesofRAD52andRAD59havenotyetbeenidentifiedinotherspecies,buttheexisteneeofRAD59homologuesinotherspeciescouldhelpexplainthediffereneeinphenotypesconferredbyRAD52ifsomeofthefunctionsofRAD52canalsobeassumedbyRAD59inS.Pombeandmammals-Anotherkeycomponentofthehomologous-recombinationmachinery,theDNA-dependentATPaseRad54p28,29,belongstotheSNF2-SWI2proteinfamily6.MembersofthisfamilyareinvolvedinmanyastsofDNAmetabolism,suchastranscription,recombinationandDNArepair.GeneticexperimentshaveshownthatRAD54-knockoutyeast,chickenandmousecellsareIR-sensitiveandhaveareducedlevelofhomologousrecombination12,30,31.Rad54pcaninteractwithRad51p32-34,andbiochemicalexperi・meritshaveshownthatRad54pstimulatesRad51pmediatedjoint-moleculeformation28.BecauseRad51pactivitycanbestimulatedbyanumberofproteins(Rad52p,Rad54pandRad55/57p),itisofinteresttodeterminetheexactstepinthestrand-exchangereactionatwhichtheseproteinsact.TheotherproteinsintheRAD52epistasisgroup,Rad50p,MrellpandXrs2p,formacomplexthatisrequiredfortheformationofmeiosis-specifiDSBs,whicharesubsequentlyrepairedthroughrecombinationwiththehomologouschromosome9・Inmitoticcells,thecomplexisprobablyinvolvedinonlyasubsetofDNArepairreactionsthatrequirehomologousrecombination35.Interestingly,recentexperimentshaveshownthatRad50p,MrellpandXrs2parealsorequiredforDNAend-joining(seebelow).DSBrepairbyDNAend-joiningTheDNAend-joiningpathwayofDSBrepairwasfirstcharacterizedinrodentcells36•UsingacollectionofChinesehamsterovarycelllinesselectedonhebasisoftheirradiosensitivity,threeDSBrepairgeneshavebeenisolated:XRCC4,XRCC5and\noODNA-PKCSSir2p/3p/4pRAD50MRE11NBS1LigaseIVXRCC4FIGURE2ModelforDNAdouble-strandbreak(DSB)repairthroughDNAend-joining.UponDSBformation,theKUheterodimerbindstotheDNAendsandattractsDNA-PK^.Inadditiontothis,chromatinstructuremightalsobeinfluenced.InSaccharomycescerevisiae,Sir2p,Sir3pandSir4pareinvolvedinthisprocess,andasimilarchromatin-remodellingreactionmightalsooccurinmammals.Subsequently,theendsarebroughttogetherandtheDNA-PKC$protomersphosphorylateeachother,causingastructuralchangeinthecomplex,possiblyresultinginremovalofDNA-PKCS.Forthelaterstagesoftherepairprocess,thecomplexcontainingRAD50,MRE11andNBS1isattracted,whichmightprocesstheends.Finally,theDNAligase-IV-XRCC4complexrejoinsthestrands.ItshouldbestressedthatitisnotknowninwhichorderthevariousproteincomplexesareattractedtotheDNAend.ThetwointerwoundDNAstrandsarerepresentedasribbons,andproteinsasspheres.Higher-orderchromatinstructureisnotindicated,althoughtheSirproteinsactbymodulatingchromatinstructurethroughinteractionswithhistones.\nreviewsTABLE1-DSBREPAIRPROTEINSANDMUTANTSOFSACCHAHOMYCtSCtRtVISIAtANDMAMMALS*SourceMutantphenotypesInteractionRemarks$・cerev/s/aiMammalianJ.cerev/s/aeMammalianRadSIpRADS1VeryIR-sensttivt11EmbryonklethaPx心<152护";RadS4pM-M#BRCA1SBRCA24SearchforDNAhomology*RadS2pRADS2VeryIR-sen$itive,2Slightlyreducedrecombination2*RadSIpStimulatesRJdS1p*A6RadS4pRAD54VeryIR-$ensitiveuIR-sensitiveEScelbwithreducedrxomb*u計on10川RadSIpStimulateRad51paRadS5/57pIR-sensithre12Rad51pStimulatesRadSIp14RADS1B-DSimilaritytoRADSI11-1*XRCC2andXRCC3DNA查看更多